 Presents
Presents
a short article on the physics of
Anodized Titanium Color
There is no dye in the colors brought out in titanium, niobium, or tantalum.
The colors are produced by controlled application of a fairly simple law of physics.
The same law that explains the rainbow colors seen in
oil on wet pavement

- When 2 parallel continuous waves of the same frequency collide, the resulting wave will be the same frequency, and somewhere between the sum of the sizes of the two incident (starting) waves, and completely flat. If the crest of one lines up with the trough of the other, they cancel out. If the peak lines up with the peak, then they reinforce.
- Light, which is made up of energy packets called photons, can also be modeled as light waves. A beam of light can be accurately visualized as waves in the (proven non-existent) interstellar ether, like water waves propagate on water.
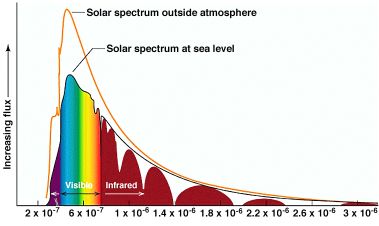
- Visible light has wavelengths from 350nm (blue) to 700nm (red). Between these falls the full rainbow spectrum of visible light.
nm = nano-meters: 25,400,000 nm = 1", 1,000 nm = 1 micron, 1/10nm = 1 Å is the diameter of a hydrogen atom.
- White light is made up of all colors together in particular proportions; similar to a black-body radiation skewed bell curve peaking at blue-green. The solar spectral distribution is what we call white.
- When there is a transparent dielectric material (dielectric = insulator, like glass, water, quartz, titania) in contact with air, light will reflect off of the boundary with an efficiency proportional to the sine of the incident angle and to the dielectric constant (density, sort of) of the material. The farther the angle is from straight on, the better the surface reflection.
- Light reflects from metals. Duh, you say?
Look at a reflection from a tight angle to a mirror. Notice how it doubles? You are seeing the reflection from both the dielectric glass, and from the metal silver
How it Works:
Okay, so we know that a clear layer on metal is like a mirror (or vice-versa).
There are really 2 reflections.
So, if a light wave of a single frequency hits the mirror, it reflects once from the clear part,
and once from the metal. When they come out, they run into each other. What happens?
Right. They interfere with each other. If the dielectric is thick enough, like several wavelengths of light or more,
nothing noticeable happens. All the interesting effects are statistically cancelled out.
However, if the distance the light has to travel through the dielectric to bounce off of the metal
and back up to where it hits the first reflection is between ½ and 2 or so times the wavelength, then
the interference gets interesting. If, for example, the total length (twice the thickness) is about half the length of the
wavelength of red light, then it is also about the full wavelength of blue light. So, blue light will be reinforced, and red will be cancelled!
Since white is made up of all colors, the blue end of the rainbow comes back out, and the red gets lost.
Voila! Color. Maybe this illustration will help:
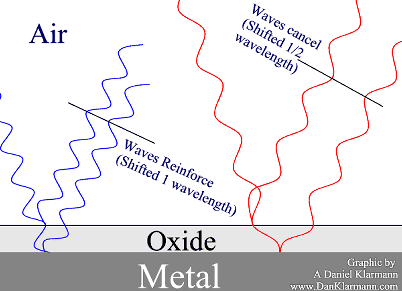
The shorter blue wavelength reflects of the surface, and off the metal.
The interior wave travels a full wavelength farther than the
first reflection, so they reinforce.
The longer red wave travels the same distance, but that's only ½ of its wavelength, so it cancels out.
When all the colors which are in white bounce off this double layer, what you see is blue.
Some more details:
- Light travels slower in a dielectric. The speed of light reduction factor is also known as the dielectric constant.
Titania (TiO2) has a fairly high constant, so the wavelength of light in the titanium surface is noticeably shorter for a given color than the vacuum-based 350-700nm. This doesn't change the way it behaves. - On the microscopic scale of wavelengths of light, the metal surface is not really flat. The oxide surface is only somewhat flatter, but not exactly parallel . This uneven-ness actually enhances the colors by statistical integration of the different colors coming from microscopically different areas and microscopically different angles. In fact, the brightest colors are realized by etching the surface to a veritable mountain range of micro-crystalline topography.
- What about green in the illustration? Well, the human eye sees red, green and blue as individual colors. All other
colors we describe are made up of combinations of these 3. Although we are taught
ROYGBIV,
in school (which shows green in the middle), green is actually very close to red in the spectrum.
Because the titanium colors are actually statistical blends of zones of the spectrum, the titanium spectrum runs:
bronze (the blue half of the spectrum is cancelled, and nothing else affected)
blues (red-green is cancelled)
blue-white (almost balanced, but still missing some red)
yellow (cancelling blue, the second time around. This is the practical high-end for heat coloring)
magenta (also called purple, cancelling green, leaving blue and red)
cyan (cancelling red more narrowly, leaving bue and green
green (cancelling both ends, keeping the narrow green band. Hard to get in Titanium, easier with Niobium.)One can, with persistence, heat color up to green, but it is quite an effort - How to produce the oxide layer and get the colors:
- Heat. Titanium eagerly grabs oxygen from the air, but the oxide layer blocks slow moving oxygen from the surface. So, torch the surface gently to give the atoms the boost they need to charge through the dielectric barrier to bond together and build a thicker layer. It's a bit hard to control, but you can easily get the first-order colors (bronze, blues, maybe yellow). Higher-temperature / longer-time colors are much harder to get and control with heat.
- Electricity: Another way to speed up those atoms is to put an electric charge on them. In an electrolytic solution
(water with a polite chemical like TSP, Borax, or Ammonium Phosphate to give the essentially dielectric liquid an ion carrier),
Colors by approx voltageput some metal (stainless, aluminum, titanium) in at one end as the negative electrode for the hydrogen to bubble off of, and connect the positive lead to your titanium piece. The higher the voltage, the harder the oxygen ions slam into the dielectric, so the thicker the dielectric grows before it can block those ions. Conveniently, the barrier potential of useful colors is between about 25 and 130 volts.

To get the higher voltage colors (magenta/pink and greens) the surface must be etched.
- Check out my anodizing page for more detailed instructions on coloring titanium with an anodizer.
- Read my Oxide calculations page for the math to figure out how thick the oxide is and how much current you need.
- See my anodizer page for instructions on building or getting an anodizer (the power supply).
- Read my titanium details page for information about the history, chemistry, and so forth of element 22

